Sci4Teens Competition: Grand Prize Winner
- cellfiemagazine

- Oct 11, 2020
- 9 min read
Alzheimer’s Disease: Neural Changes and the Pre-Clinical Gap - Amy Lynn Hutchinson
Abstract
Alzheimer’s Disease (AD) is the most common type of Dementia and the sixth leading cause of death in the US. Despite this heavy disease burden, there are no proven methods for preventing Alzheimer’s. Scientists have identified several brain changes vital to the development of AD, including accumulation of Amyloid Plaques, Neurofibrillary Tangles, and Brain Inflammation. Interestingly, scientists have observed that some people can develop the biological structures of Alzheimer’s, but none of the symptoms of memory loss or cognitive decline. This has led to questions about this “pre-clinical gap” and why people with similar brain structures can have different cognitive functions. One attempt to explain this is the combined theories of Cognitive Reserve (the brain’s ability to adapt to change), Brain Reserve (number of available brain structures) and Brain Mechanisms (ability to maintain structures) which may explain how the brains responds to disease and provide valuable prevention targets.
What is Alzheimer’s?
The clinical presentation (symptoms observed in a doctor’s office) of Alzheimer’s first develops as an inability to remember newly learned information, like new names or places, as the learning center of the brain begins to deteriorate. As the disease worsens disorientation, mood and behavior changes (like anxiety, wandering, and emotional outbursts), increasing confusion (patient not knowing where they are, etc.), and serious memory loss (unable to remember familiar names, etc.) develop. Eventually, difficulty speaking, walking, and swallowing may onset, as the brain’s motor regions are the final areas affected by the disease (What Is Alzheimer’s Disease? Symptoms & Causes | Alz.Org). The base cause of these symptoms is neurodegeneration (the death of neurons, which are the brain’s cells). This neuron death is caused by several structural and physiological abnormalities that degrade neuron functioning. These abnormalities are found in healthy individuals and those with other kinds of dementia, but in Alzheimer’s patients they are much more prevalent and form patterns specific to the disease.
Neurobiology of Alzheimer’s: Beta-Amyloid Plaques
The first brain abnormalities to develop are Beta-Amyloid Plaques. These are small bunches of proteins that build up around cells, the same way plaque builds up on teeth. These plaques are made from a protein that is normally “cut” into small pieces by enzymes. When the enzymes malfunction, the protein isn’t cut correctly, and thus forms long strands that can’t be broken down by the brain. These long pieces kill the cells by piling up around them and preventing cellular communication (What Is Alzheimer’s Disease? Symptoms & Causes | Alz.Org).
While plaques form first in an AD patient, the correlation between the amount of plaque build-up and a person’s level of cognitive decline is actually quite low (Mufson et al.). It is not known why this is, but one theory states that the makeup of and way the plaques are deposited is more important to AD progression. This is supported by the fact that studies have found a higher correlation between cognitive decline and plaques that are both less soluble and form larger pools (Murphy and Iii). Others argue that plaques have little effect on a person’s cognition and instead trigger later brain developments that more profoundly impact a person’s functioning. More research is being conducted in this area to try and answer these important questions.
Neurofibrillary Tangles
The second brain abnormalities to develop are called Neurofibrillary Tangles. These are long
strands of what are called Tau Proteins. Tau normally make up the cell’s “skeleton” and provide structure and transport pathways for the cell. In people with Alzheimer’s, Tau proteins in the brain undergo a process that adds a chemical “tag” (called a phosphate group) onto them. This tag degrades the tau proteins in the cell’s skeleton, causing the skeleton to collapse and the proteins to clump together into long bundles (or “tangles”) inside the cell (Ebneth et al.). As the cell’s structure breaks down it loses its functioning and dies (Kinney et al.; Amyloid Plaques and Neurofibrillary Tangles | BrightFocus Foundation). Tangles develop later in the progression of AD, and appear to have a greater impact on a person’s cognitive functioning, as a greater prevalence of tangles better correlates to a loss of cognitive abilities (Mufson et al.).
Inflammation
For a long time, it was not understood how plaques and tangles related to each other, and if there was any relationship between them at all. Some argued that plaques caused tangles, while others believed that they developed completely on their own irrespective of each other. Recently, scientists have identified a potential link between these two features that may help explain how Alzheimer’s develops.
Inflammation is a natural part of the body’s immune response. It appears that the presence of plaques in the brain “turns on” the immune system and the brain becomes inflamed as the body reacts to plaques the same way it reacts to foreign bodies like a viruses or allergens. While inflammation is normally a healthy part of the immune system it may prove to be harmful for AD patients.
Early in the disease the immune response is helpful, as immune cells are able to break down
plaques. But these immune cells eventually (and for an unknown reason) lose their ability to
degrade plaques, and because the body still detects a foreign agent the immune cells still secrete pro-inflammatory chemicals that keep the inflammation cycle going (Kinney et al.).This chronic inflammation harms the brain, as neurons are degraded by the presence of immune cells and the chemicals they release. Thus, inflammation contributes to symptom-causing neurodegeneration (Sartori et al.).
Cycle of Chronic Inflammation in Alzheimer’s Patients
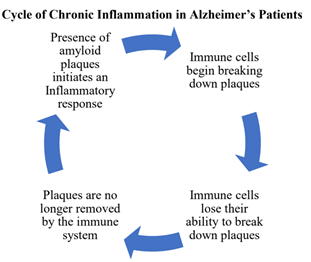
As the immune system responds to the plaques, it not only leads to neuron death, but may
provide a key link between plaques and tangles. Chronic inflammation in the brain may actually drive the development of tangles by activating enzymes that add phosphates (the chemical “tag”) onto Tau proteins (Kinney et al.). Thus, plaques may indirectly cause tangles by initiating brain inflammation.
Overview of the Alzheimer’s Disease Biological Cascade

The Pre-Clinical Gap
While it is known that symptoms of AD are caused by neuron death, some patients may have a surprising resistance to cognitive decline. Researchers have become increasingly aware of a “pre-clinical” phase of AD, where a person has the biological features of the disease (plaques, tangles, inflammation, and neurodegeneration) but no clinical symptoms. It has been estimated that this pre-clinical phase can last for many years and even decades, with one study finding AD structures in 25-67% of cognitively normal patients (Dubois et al.; Scarmeas and Stern). There is much that not understood about this pre-clinical stage, with some arguing that it is only a slowed progression of AD that will eventually develop, and others saying that it is a sign of AD “resistance” (Abner et al.; Scarmeas and Stern; Vos et al.). Despite the many unknowns it can be said for certain that understanding this “pre-clinical gap” could help unlock powerful methods of preventing Alzheimer’s.
Theories of Alzheimer’s Cognition
There are several complex factors that play a role in the physical-clinical gap, and a recent theory may help explain why people can have significant neuron breakdown while maintaining full cognitive functions.
The first important concept is called Brain Reserve. This refers to the amount and healthiness of the brain structures (like neurons and synapses) someone has. To use an analogy, if the brain is a computer then Brain Reserve is the hardware the user has to work with (Stern et al.). There is a certain level of neuron death required for cognitive deficits to occur; what can be called a “cognitive threshold”. Thus, someone with a higher brain reserve may simply have more brain structures to lose. A higher brain reserve could help stave off clinical symptoms as the individual reaches the cognitive threshold later in life, potentially never. Brain reserve is still not well defined, it is likely that many genetic, health, and environmental factors play a role in how much an individual has (Stern et al.)
The second concept is Brain Mechanism, which is the brain’s ability to maintain the structures
of the Brain Reserve and resist losing them by making “repairs”. Essentially, Brain Mechanisms are the technician fixing the computer’s hardware to help keep the software running. (Stern et al.)
The last, and perhaps most interesting component, is Cognitive Reserve. Cognitive reserve is the term for how well the brain adapts to losing structures by finding new ways of performing functions when the usual pathways have been degraded (What Is Cognitive Reserve? - Harvard Health). So, while people may still develop the biology of AD, their brains can maintain cognitive functions by finding ways to “work around” structural loss. Thus, two people could have the same neurodegeneration but very different symptoms, as one brain is better able to adapt to the degeneration. Cognitive reserve can be compared to the software on a computer, the better the software the better the computer can run as a whole (Stern et al.).
Cognitive Reserve and AD Prevention
Given the important role cognitive reserve may play in AD development, strengthening it could help slow the disease’s progression.
A common concept is that of “keeping the brain active”, an idea often partnered with things like games or apps meant to “exercise” the brain. But building and maintaining cognitive reserve may be much simpler than that.
Consistently, studies have found that participating in household, leisure, and social activities
decreases risk for dementias (including Alzheimer’s). Leisure activities like board games,
reading, knitting, and gardening have been shown to have positive impacts on cognitive abilities in late life. Being involved in community events, taking classes, and having a strong social network have also been identified as protective factors (Scarmeas and Stern). Even participating in daily activities like bookkeeping, managing medications, and looking after household needs has been shown to have a small but significant effect on preserving cognitive functions (NIH Alzheimer’s and Cognitive Decline Prevention Conference - Panel
Statement). It has been proposed that these activities help strengthen cognitive functions by stimulating neurons in key brains regions. This stimulation helps increase the number of synapses (space neurons communicate, illustrated left) which could improve the efficiency of brain systems by making each neuron better able to communicate. This new efficiency could help the brain better compensate for lost neurons (Scarmeas and Stern)

Synapses and Neuron Communication
Neurotransmitters move through the synapse and allow
neurons to communicate (Wikimedia)
Conclusion
Alzheimer’s is more than a set of clinical presentations; it is a complex series of brain functions and malfunctions. Understanding how the brain changes in order to create the symptoms (or work around them) will allow scientists to pinpoint ways to slow or prevent the development of AD. Currently, there are no effective therapies that target biological features like plaques, tangles, and inflammation which makes prevention and the theories of cognitive reserve, brain reserve, and brain mechanism, all the more important. Understanding the pre-clinical gap may provide the best chance for lowering the Alzheimer’s disease burden by offering insights into AD prevention. Next steps in Alzheimer’s research should focus on working to identify anatomical differences in the brains of those who remain in the pre-clinical phase of AD, and those who develop more severe stages. Comparing the brain anatomy and functioning of these two groups could help researchers understand how the brain is able to fend off cognitive decline. Studying the lifestyles and biology of those individuals could then provide concrete steps to help people slow or prevent the onset of Alzheimer’s. Altogether leading to preventative measures that could change millions of lives.
References
Abner, Erin L., et al. “‘End-Stage’ Neurofibrillary Tangle Pathology in Preclinical Alzheimer’s
Disease: Fact or Fiction?” Journal of Alzheimer’s Disease, vol. 25, no. 3, IOS Press, Jan.
2011, pp. 445–53, doi:10.3233/JAD-2011-101980.
Amyloid Plaques and Neurofibrillary Tangles | BrightFocus Foundation.
neurofibrillary- tangles. Accessed 18 Aug. 2020.
Dubois, Bruno, et al. “Preclinical Alzheimer’s Disease: Definition, Natural History, and
Diagnostic Criteria.” Alzheimer’s and Dementia, vol. 12, no. 3, Elsevier Inc., 1 Mar. 2016,
pp. 292–323, doi:10.1016/j.jalz.2016.02.002.
Ebneth, A., et al. “Overexpression of Tau Protein Inhibits Kinesin-Dependent Trafficking of
Vesicles, Mitochondria, and Endoplasmic Reticulum: Implications for Alzheimer’s
Disease.” Journal of Cell Biology, vol. 143, no. 3, The Rockefeller University Press, Nov.
1998, pp. 777–94, doi:10.1083/jcb.143.3.777.
Kinney, Jefferson W., et al. “Inflammation as a Central Mechanism in Alzheimer’s Disease.”
Alzheimer’s and Dementia: Translational Research and Clinical Interventions, vol. 4,
Elsevier Inc, 1 Jan. 2018, pp. 575–90, doi:10.1016/j.trci.2018.06.014.
Mufson, Elliott J., et al. “Molecular and Cellular Pathophysiology of Preclinical Alzheimer’s
Disease.” Behavioural Brain Research, vol. 311, Elsevier B.V., 15 Sept. 2016, pp. 54–69,
doi:10.1016/j.bbr.2016.05.030.
Murphy, M. Paul, and Harry Levine Iii. “Alzheimer’s Disease and the β-Amyloid Peptide NIH
Public Access.” J Alzheimers Dis, vol. 19, no. 1, 2010, p. 311, doi:10.3233/JAD-2010-
1221.
NIH Alzheimer’s and Cognitive Decline Prevention Conference - Panel Statement.
https://consensus.nih.gov/2010/alzstatement.htm. Accessed 19 Aug. 2020.
Sartori, Andrea C., et al. “The Impact of Inflammation on Cognitive Function in Older Adults:
Implications for Healthcare Practice and Research.” Journal of Neuroscience Nursing,
vol. 44, no. 4, NIH Public Access, Aug. 2012, pp. 206–17,
doi:10.1097/JNN.0b013e3182527690.
Scarmeas, Nikolaos, and Yaakov Stern. “Cognitive Reserve: Implications for Diagnosis and
Prevention of Alzheimer’s Disease.” Current Neurology and Neuroscience Reports, vol.
4, no. 5, Current Science Ltd, 2004, pp. 374–80, doi:10.1007/s11910-004-0084-7.
Stern, Yaakov, et al. “Whitepaper: Defining and Investigating Cognitive Reserve, Brain
Reserve, and Brain Maintenance.” Alzheimer’s and Dementia, Elsevier Inc., 2018,
doi:10.1016/j.jalz.2018.07.219.
Vos, Stephanie J. B., et al. “Preclinical Alzheimer’s Disease and Its Outcome: A Longitudinal
Cohort Study.” The Lancet Neurology, vol. 12, no. 10, Elsevier, Oct. 2013, pp. 957–65,
doi:10.1016/S1474-4422(13)70194-7.
What Is Alzheimer’s Disease? Symptoms & Causes | Alz.Org. https://www.alz.org/alzheimers-
dementia/what-is-alzheimers. Accessed 3 Aug. 2020.
What Is Cognitive Reserve? - Harvard Health. https://www.health.harvard.edu/mind-and-
mood/what-is- cognitive-reserve. Accessed 19 Aug. 2020.
Wikimedia. Depiction of Neuronal Synapse. 2011,






Comments